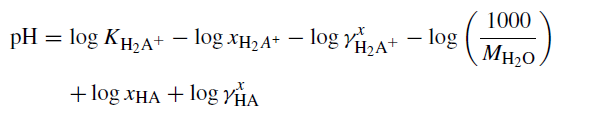Calculation of pH in multiple solvents
Is there a general pH equation for multiple solvents?
A user asked the question, "Can the OLI pH equation for the Mixed-Solvent-Electrolyte model be extended to multiple solvents?"
The treatment of pH in mixed-solvent-electrolyte systems has been described at great length in the paper " MSE Acid-Base Equilibria 2007 (PDF)". Specifically we need to consider equation (13) in that paper:
Andre Anderko, PhD, FNACE (CTO, OLI Systems) commented:
This equation is all that we can do considering the current state of the science.
Let me explain. There is no universal pH scale that would work for all protic solvents. Therefore, IUPAC officially recommends separate scales for each solvent. Separate scales would be of course rigorous but of little practical use. The reason lies in the experimental reality. In general, pH is measured as a response of an electrode (usually a glass electrode but not necessarily) to some species that can cross the solution/electrode barrier and undergo an interfacial reaction. Those species are the protons (or hydronium ions) in aqueous systems and various protonated forms of other solvents in other systems. The experimental fact is that all these species are transferred somewhat differently across the interface, which gives rise to a different response. So, there is no common scale and formal thermodynamics is of no help.
Therefore, we investigated the situation on an empirical basis. We made a hypothesis that most protonated species (at least the ones that are small enough to make it across the glass electrode interface) should contribute to the electrode response in pH measurements. In other words, there may be various carriers of protons that can deliver the protons to the electrode. We tested this hypothesis on H2O2 systems and it worked. This was a “Eureka” moment and we published it in our 2007 paper. This meant that we had to add the concentrations of the proton-bearing species, in this case protons and protonated H2O2. Then, we applied the same method to MEG system, which works based on summing up the hydronium ions and the protonated form of MEG. Since we are summing up concentrations, we cannot account for activity coefficients (because activities are not additive, only concentrations may be added up).
So, the current state of the art is as follows:
- If water is the only solvent, eq. 13 is a rigorous way of calculating pH
- If there are other solvents, there is no rigorous way to calculate pH (and many people would say that there will never be a rigorous way). However, our approximate method based on adding the concentrations of protonated forms of all solvents has been proven to agree with experimental measurements at least for H2O2 and MEG mixtures.