Difference between revisions of "Alkalinity and OLI"
| Line 27: | Line 27: | ||
The amount of HCl added to bring the pH down to 4.5 is: | The amount of HCl added to bring the pH down to 4.5 is: | ||
| + | |||
| + | [[File:Table 3.jpg]] | ||
| + | |||
| + | '''Figure 3 215.4 mg/L of HCl required.''' | ||
| + | |||
| + | This amount of HCl needs to be converted to equivalents of bicarbonate ion for reporting purposes. | ||
| + | |||
| + | [[File:Formula 1.jpg]] | ||
Revision as of 11:08, 11 July 2016
What does OLI mean when it discusses Alkalinity? Alkalinity is a frequently measured and reported quality of many waters. Stumm and Morgan define alkalinity as:
“Acidimetric or alkalimetric titrations of carbonate-bearing water to the appropriate end points represent operations procedures for determining alkalinity and acidity, that is, the equivalent sum of the bases that are titratable with strong acid and the equivalent sum of the acids that are titratable with strong base. Alkalinity and acidity are then the capacity factors that represent, respectively, the acid- and base-neutralizing capacities … of an aqueous system. For solutions that contain no protolysis system other than that of aqueous carbonate, alkalinity is a measure of the quantity of strong acid per liter required to attain a pH equal to that of a total concentration (molar) solution of H2CO3. Alternatively, acidity is a measure of the quantity per liter of strong base required to attain a pH equal to that of a total concentration (molar) solution of Na2CO3”1
The key to this statement involves the fact that many users think that the alkalinity is the concentration of various forms of carbonate ion. This would be true of other acid or base systems were not present in solution. Even simple ions such as sodium and magnesium may affect the free carbonate in solution and have markedly different alkalinities.
OLI considers alkalinity to be the total base capacity of the brine. We will us a titration to determine the alkalinity exactly. We will now show some examples featuring the OLI/LabAnalyzer™ program.
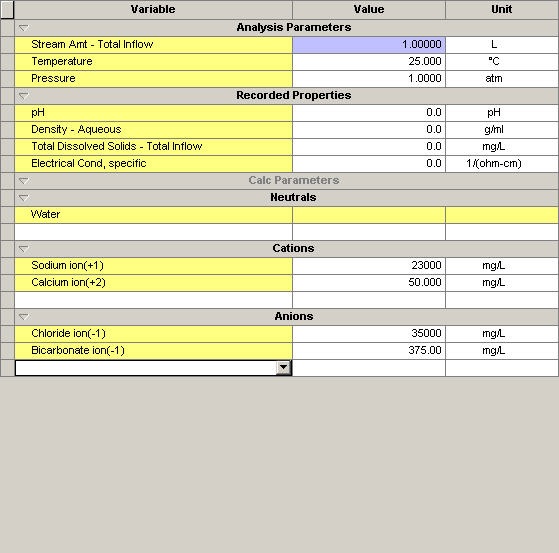
We will consider a simple brine with the following concentration
1“Aquatic Chemistry. An Introduction Emphasizing Chemical Equilibria in Natural Waters”. Werner Stumm and James J. Morgan. John-Wiley & Sons, New York. 1981 p 185
Figure 1 Brine composition.
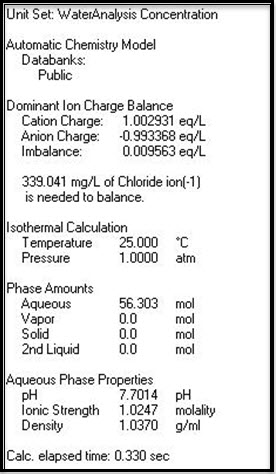
The user would suspect that the alkalinity would be the same as the bicarbonate concentration of 375 mg/L. The electrically neutral pH of this brine is:
Figure 2 the pH is 7.7
We will use HCl to titrate the brine to the standard end point pH of 4.5.
The amount of HCl added to bring the pH down to 4.5 is:
Figure 3 215.4 mg/L of HCl required.
This amount of HCl needs to be converted to equivalents of bicarbonate ion for reporting purposes.