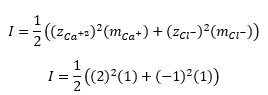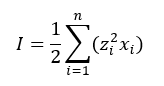Difference between revisions of "Ionic Strength"
(→Converting from Mole fraction to Molal-based units) |
(→Converting from Mole fraction to Molal-based units) |
||
| Line 32: | Line 32: | ||
The equation to use is the following: | The equation to use is the following: | ||
| − | + | [[File:Ionic Strength Conversion.png]] | |
Where: | Where: | ||
| − | + | I_m = ionic strength in molal-based units <br> | |
| − | + | I_X = ionic strength in mole fraction-based units<br> | |
LIQMOL = moles of liquid (true species)<br> | LIQMOL = moles of liquid (true species)<br> | ||
| − | + | GMOLH2O = moles of water in the liquid phase (true species)<br> | |
[[user:DMILLER | Author: Diana Miller]] | [[user:DMILLER | Author: Diana Miller]] | ||
Revision as of 07:50, 7 September 2022
Ionic Strength (molal based or m-based)
The ionic strength is a quantity representing the strength of the electric field in a solution, and it is equal to the sum of the molalities of each type of ion present multiplied by the square of their charges, as represented by the following equation:
Where n is the number of charged species.
For example, a 1.0 molar solution of NaCl has 1.0 moles of Na^+ions and 1.0 moles of Cl^- ions in 1 kg of H2O. Therefore, the ionic strength is 1.0 molal.
Now, consider a 1.0 molal solution of CaCl2. This solution has 1.0 moles of Ca^(+2) ions and 2.0 moles of Cl^- ions in 1 kg of H2O. Therefore, the ionic strength is 3.0 molar, or it can be said that a 1.0 molal solution of CaCl2 behaves similar to a 3.0 molar solution of NaCl.
Ionic Strength (mole fraction based or x-based)
In this case the ionic strength is calculated using the mole fraction rather than the molality:
Where n is the number of charged species.
Converting from Mole fraction to Molal-based units
The equation to use is the following:
Where:
I_m = ionic strength in molal-based units
I_X = ionic strength in mole fraction-based units
LIQMOL = moles of liquid (true species)
GMOLH2O = moles of water in the liquid phase (true species)