Difference between revisions of "Modeling the Chemistry of Carbon Dioxide - Rich Phases with Impurities"
| Line 1: | Line 1: | ||
| − | + | '''Dense Phase CO2 Corrosion: Modeling the Chemistry of CO<sub>2</sub> – Rich Phases with Impurities''' | |
| + | |||
| + | |||
| + | == Objectives: == | ||
| − | |||
*: - Model the solubility of S<sup>o</sup> in CO<sub>2</sub> - rich phases in order to predict whether solid S<sup>o</sup> can drop out in transmission lines | *: - Model the solubility of S<sup>o</sup> in CO<sub>2</sub> - rich phases in order to predict whether solid S<sup>o</sup> can drop out in transmission lines | ||
*: - Predict whether S<sup>o</sup> can undergo reactions in the presence of water | *: - Predict whether S<sup>o</sup> can undergo reactions in the presence of water | ||
| − | + | ||
| + | == Chemical subsystems to be modeled == | ||
| + | |||
*: - Pure S<sup>o</sup> | *: - Pure S<sup>o</sup> | ||
*:: Volatility of pure S<sup>o</sup> provides a baseline for its solubility in gas phase | *:: Volatility of pure S<sup>o</sup> provides a baseline for its solubility in gas phase | ||
| Line 17: | Line 21: | ||
*:: Will be important for future modeling of reactions of SOx and NOx | *:: Will be important for future modeling of reactions of SOx and NOx | ||
| − | + | ||
| + | == Polymeric species of sulfur == | ||
| + | |||
*: - Numerous sulfur species up to S<sub>20</sub> have been detected | *: - Numerous sulfur species up to S<sub>20</sub> have been detected | ||
*: - In the gas phase, eight multimers (S<sup>o</sup><sub>1</sub> through S<sup>o</sup><sub>8</sub>) have been assumed in the model | *: - In the gas phase, eight multimers (S<sup>o</sup><sub>1</sub> through S<sup>o</sup><sub>8</sub>) have been assumed in the model | ||
| Line 32: | Line 38: | ||
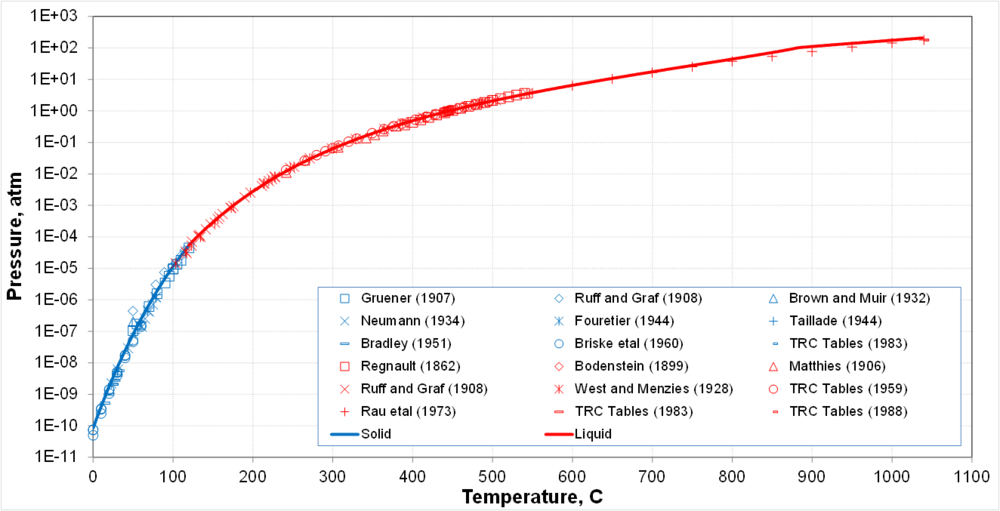
* Solid-vapor equilibrium transitions into liquid-vapor equilibrium at the triple point | * Solid-vapor equilibrium transitions into liquid-vapor equilibrium at the triple point | ||
* Calculations are supported by a large body of generally consistent data | * Calculations are supported by a large body of generally consistent data | ||
| + | |||
| + | '''Melting point of S<sup>o</sup>'''<br> | ||
| + | [[File:02.png| 1000 px | boarder]] | ||
| + | |||
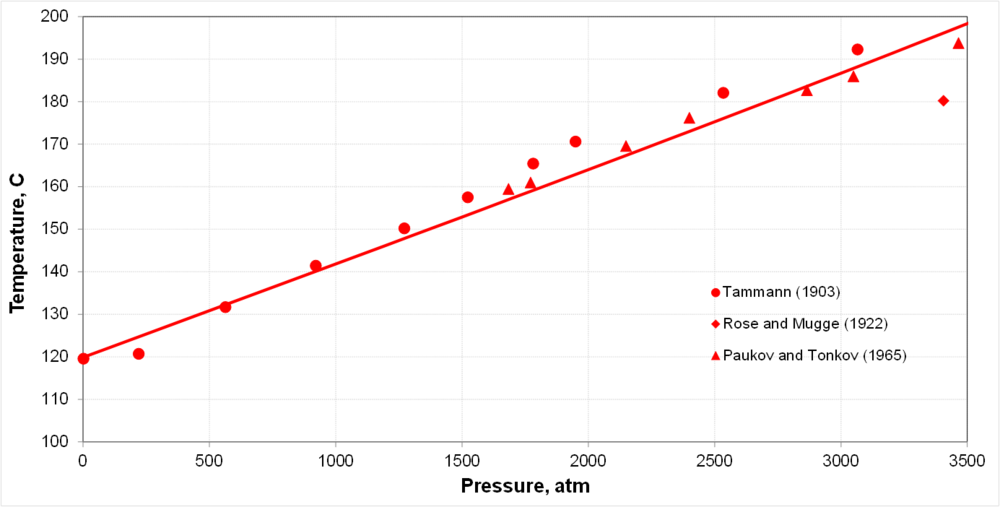
| + | * Pressure significantly affects the melting point | ||
| + | |||
| + | '''Solubility of S<sup>o</sup> in water'''<br> | ||
| + | [[File:03.png | 1000px | boarder]] | ||
| + | |||
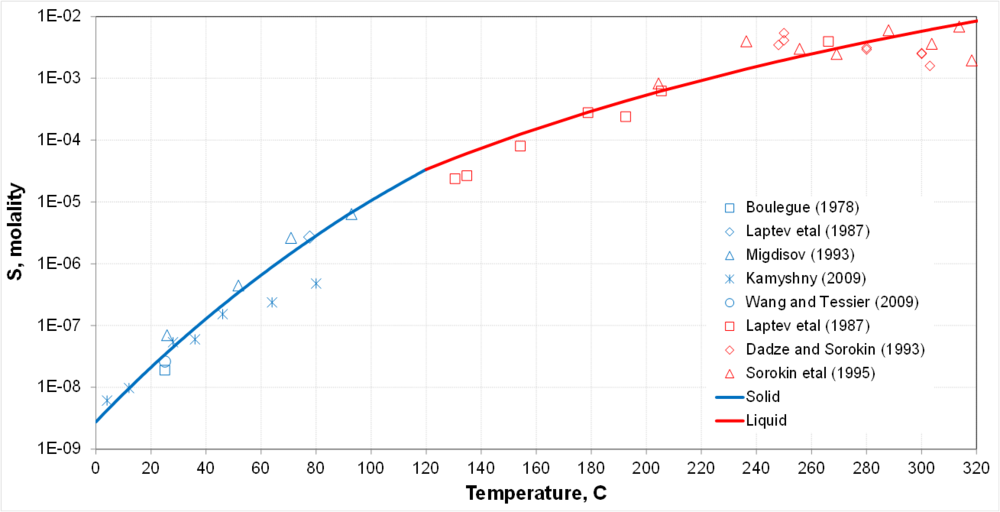
| + | * Transition between solid-liquid equilibria and liquid-liquid equilibria at the melting point of sulfur | ||
| + | * Solubility is a strong function of temperature | ||
| + | |||
| + | '''Solubility of S<sup>o</sup> in CO<sub>2</sub>: Preliminary results'''<br> | ||
| + | [[File:04.png | 1000px | boarder]] | ||
| + | |||
| + | * Two segments of solubility curves at each T corresponding to gas (or gas-like) and liquid (or liquid-like) CO<sub>2</sub> | ||
| + | * Transition is sharp for subcritical CO<sub>2</sub> and gradual for supercritical CO<sub>2</sub> | ||
| + | * Fair amount of scattering in the data but the trends are clear | ||
| + | * In scCO<sub>2</sub>, changes due to SVE-SLE transitions are obscured by uncertainty in data | ||
| + | |||
| + | |||
| + | == Modeling sulfur redox == | ||
| + | |||
| + | * Provides a starting point for modeling reactions involving sulfur | ||
| + | * Redox states | ||
| + | *: - Oxidation states range from S<sup>2-</sup> through S<sup>8+</sup> | ||
| + | *: - Practically important states: | ||
| + | *:: S<sup>2</sup>- (sulfides, hydrogen sulfide) | ||
| + | *:: S<sup>0</sup> | ||
| + | *:: S<sup>2+</sup> (thiosulfate) | ||
| + | *:: S<sup>4+</sup> (sulfite) | ||
| + | *:: S<sup>6+</sup> (sulfate) | ||
| + | *:: Thiosulfates and sulfites are usually metastable as evidenced by E-pH diagrams | ||
| + | *Experimental data are often kinetically constrained | ||
| + | *: - Metastable species may be identified in addition to stable ones | ||
| + | |||
| + | '''Sulfur redox: Preliminary results for disproportionation'''<br> | ||
| + | [[File:05.png | 1000px | boarder]] | ||
| + | * Disproportionation reactions proceed more readily at high T | ||
| + | * This is not a direct comparison because measurements are kinetically constrained and calculations predict equilibria | ||
| + | * Therefore, only semi-quantitative agreement can be expected | ||
| + | * The properties of thiosulfate species (S<sup>2+</sup>) need to be reexamined | ||
| + | |||
| + | |||
| + | == Deployment plan == | ||
| + | * Upcoming update of the OLI software | ||
| + | *: - Version 9.3.1 is in the final stage of development | ||
| + | *: - It will contain the recently developed sulfur chemistry | ||
| + | *: - Members will be able to download the new version from the OLI download site | ||
| + | |||
| + | [[User:jberthold | Author: Ron Springer (OLI), Andre Anderko (OLI), Jim Berthold (OLI, editor)]] | ||
| + | |||
| + | [[category: Thermodynamics]] | ||
Revision as of 10:16, 4 November 2016
Dense Phase CO2 Corrosion: Modeling the Chemistry of CO2 – Rich Phases with Impurities
Contents
Objectives:
- - Model the solubility of So in CO2 - rich phases in order to predict whether solid So can drop out in transmission lines
- - Predict whether So can undergo reactions in the presence of water
Chemical subsystems to be modeled
- - Pure So
- Volatility of pure So provides a baseline for its solubility in gas phase
- - So – H2O
- Solubility of So in H2O is important if an aqueous phase forms
- Model development requires considering the So – H2O system because the reference state for liquid-phase species is infinite dilution in water
- - So – CO2
- Solubility of So in CO2
- - Redox reactions of sulfur in aqueous media
- Will be important for future modeling of reactions of SOx and NOx
- - Pure So
Polymeric species of sulfur
- - Numerous sulfur species up to S20 have been detected
- - In the gas phase, eight multimers (So1 through So8) have been assumed in the model
- Thermochemical data are well established for So1 through So8
- So8 is dominant at normal and moderate conditions
- Lower multimers become prevalent at higher temperatures
- - In the pure liquid and solution phase, So8 predominates
- - In the CO2 phase, solvated So-CO2 species may appear
- Solid-vapor equilibrium transitions into liquid-vapor equilibrium at the triple point
- Calculations are supported by a large body of generally consistent data
- Pressure significantly affects the melting point
- Transition between solid-liquid equilibria and liquid-liquid equilibria at the melting point of sulfur
- Solubility is a strong function of temperature
Solubility of So in CO2: Preliminary results
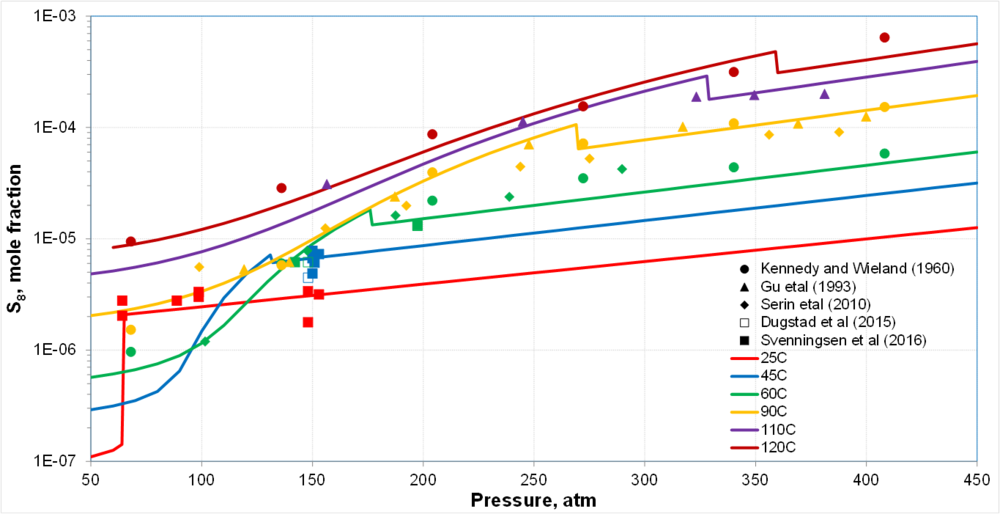
- Two segments of solubility curves at each T corresponding to gas (or gas-like) and liquid (or liquid-like) CO2
- Transition is sharp for subcritical CO2 and gradual for supercritical CO2
- Fair amount of scattering in the data but the trends are clear
- In scCO2, changes due to SVE-SLE transitions are obscured by uncertainty in data
Modeling sulfur redox
- Provides a starting point for modeling reactions involving sulfur
- Redox states
- - Oxidation states range from S2- through S8+
- - Practically important states:
- S2- (sulfides, hydrogen sulfide)
- S0
- S2+ (thiosulfate)
- S4+ (sulfite)
- S6+ (sulfate)
- Thiosulfates and sulfites are usually metastable as evidenced by E-pH diagrams
- Experimental data are often kinetically constrained
- - Metastable species may be identified in addition to stable ones
Sulfur redox: Preliminary results for disproportionation
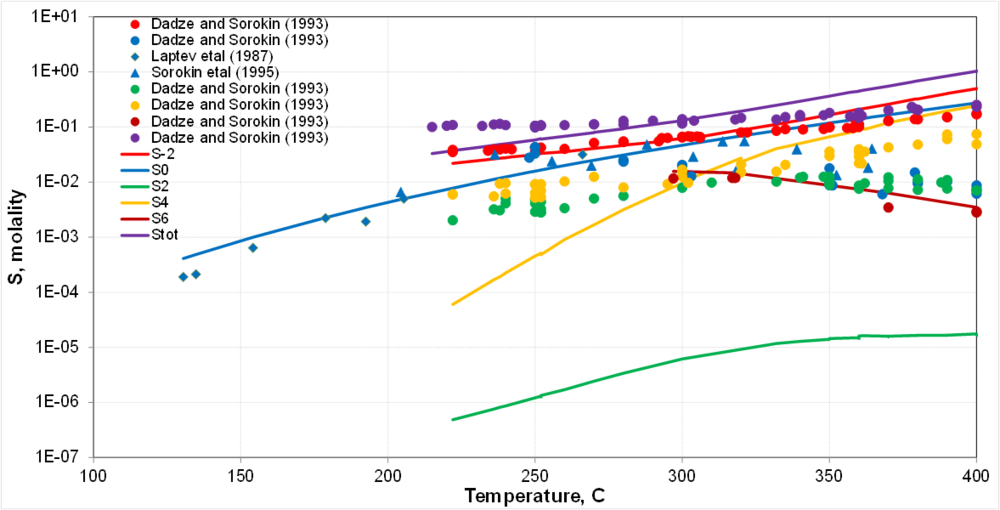
- Disproportionation reactions proceed more readily at high T
- This is not a direct comparison because measurements are kinetically constrained and calculations predict equilibria
- Therefore, only semi-quantitative agreement can be expected
- The properties of thiosulfate species (S2+) need to be reexamined
Deployment plan
- Upcoming update of the OLI software
- - Version 9.3.1 is in the final stage of development
- - It will contain the recently developed sulfur chemistry
- - Members will be able to download the new version from the OLI download site
Author: Ron Springer (OLI), Andre Anderko (OLI), Jim Berthold (OLI, editor)