Difference between revisions of "Simulating evaporation"
m (RNIMKAR moved page Simulating evaporation using a Survey calculation to Simulating evaporation without leaving a redirect) |
|||
| Line 33: | Line 33: | ||
== Evaporation in an open system == | == Evaporation in an open system == | ||
| + | |||
| + | [[Category: Simulation Techniques]] | ||
Latest revision as of 21:03, 15 October 2014
Evaporation in a closed system
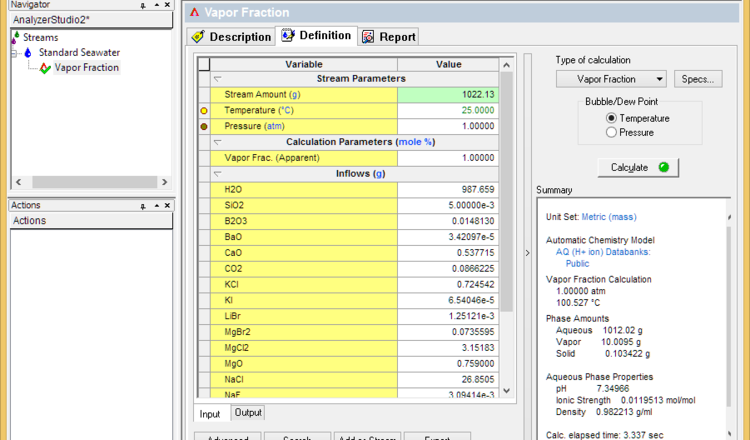
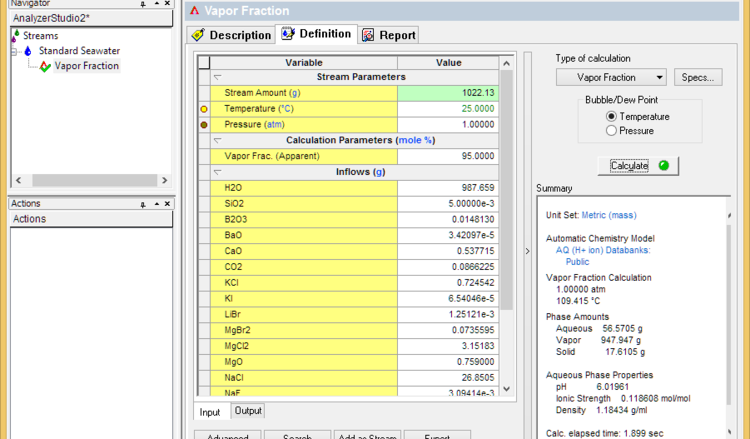
A liquid in a closed system will not start to evaporate until its boiling point is reached. Further evaporation will occur when the temperature increases. Therefore, the fastest way to simulate evaporation is using a Temperature survey. We will use Standard Seawater to present this method.
- Add a single point calculation
- Change the calculation type to Vapor Fraction
- Set the vapor fraction to 1% and calculate. This will determine the initial boiling point
- Set the vapor fraction to 99% less the % of dissolved solids (seawater for example contains about 3.6% solids). If the calculation fails, then lower the vapor fraction until it converges. This sets the dryness temperature
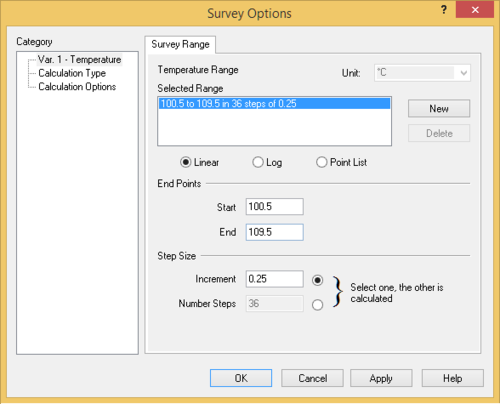
- Add a Survey calculation - Use the default Temperature survey
- Set the initial and final temperatures to the values obtained above. Add sufficient increments (e.g., 10-20) so that the plot has good resolution.
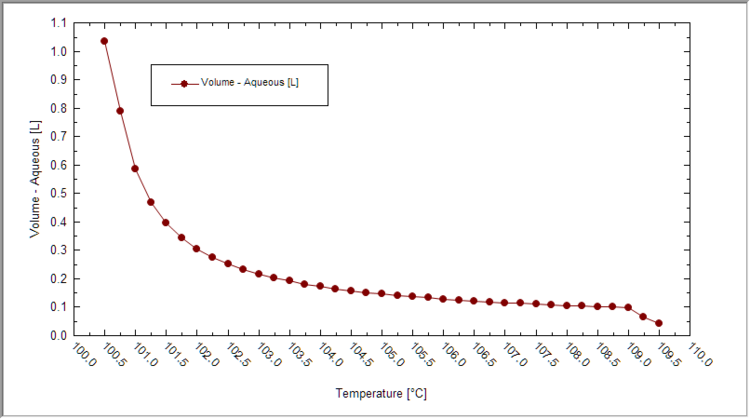
- Plot the liquid volume vs. temperature first. See if sufficient liquid evaporated for your case.
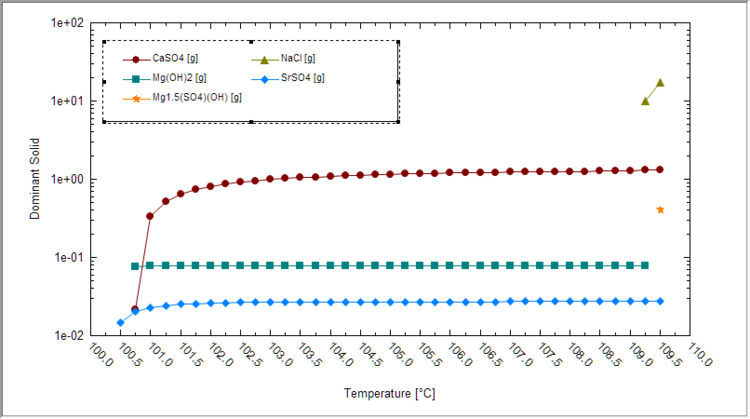
Next, plot the dominant solids vs. temperature to see which solids are forming and at what condition. It is best to view this plot using a logarithmic Y-axis