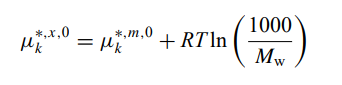Why is the Gibbs Free Energy of Formation for the Hydrogen Ion not zero?
OLI has been predicting the Gibbs Free Energy of Formation of species for some time and can report them from the software. For OLI Studio click here to see how to do that How to enable the optional calculations in OLI Studio?
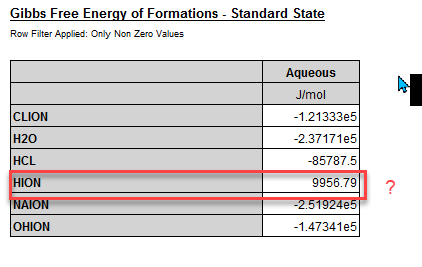
For a simple case at 25 oC we have this in our extended properties for Gibbs Free Energy in the Standard State:
The Gibbs Free Energy of Formation for the H+ should be zero. Here we are calculating the value as 9956.79 J/Mol. It is in various thermodynamic textbooks, so why is OLI calculating something different?
The difference in the standard state Gibbs free energy for species (e.g. H+) in OLI report from what is usually reported in the literature (for H+ is zero by definition) is due to different concentration scales, i.e.,
where superscript m is for molal based G0 whereas superscript x is mole-fraction based G0. OLI reports the x-based value, thus, at 25 oC and 1 atm, G0,m(H+)=0, G0,x(H+)=0+RT*ln(55.50825)=9956.8 (J/mol) (R=8.31446, T=298.15).
Our 2002 paper (Fluid Phase Equilibria 203 (2002) 141–176) has a description of the conversion.