Corrosion FAQ's
When standard database predicts precipitation of Fe(III), Zn(II), Cu(II) as Fe(OH)3, Zn(OH)2 and Cu(OH)2 respectively, Inclusion of the Corrosion database produces the more stable hematite Fe2O3, goethite FeO(OH), ZnO and CuO solids.
When you include a corrosion data bank, surely it predicts more stable oxides. That is because when a corrosion databank is included, the solver automatically assumes a surface rather than a simple solid precipitation. And then the speciation changes. The hydroxides are still considered a part of the system, but the possibility of oxides forming increases because there will be corroding surface involved in that system. Thus the stable oxide predictions.
When to use Corrosion and Geochemical databank
When you use OLI software, there are certain calculations that need additional databanks to be included by users for specific predictions. Geochemical i.e GEMSE and Corrosion, i.e. CRMSE are one of them.
There is a fine difference between scenarios which govern the fact that the databanks should be included or not. One of the major factor controlling it is time. Would a particular species be formed under specific conditions or it could only be formed over a significant period of time.
For GeoChem the controlling parameter is only time. If a component will only form over a geochemical scale then the user may include the databank to predict the formation of the component in that particular process simulation. Please remember that under ordinary circumstances this component/ solid would not be a part of that process and would not be predicted as a part of the system for that process via simulation.
A good example for this is the SiO2 system. OLI recently worked on this system. Now SiO2 and its two forms
-> SiO2 ( Amorphous)
-> SiO2 ( Quartz)
are included in MSE and GEMSE databanks respectively. Now under normal circumstances, one would expect the amorphous silicon dioxide to be present in a system. But when geological scale is involved, then it becomes necessary to include the Quartz structure, and then the geochemical databank comes into picture.
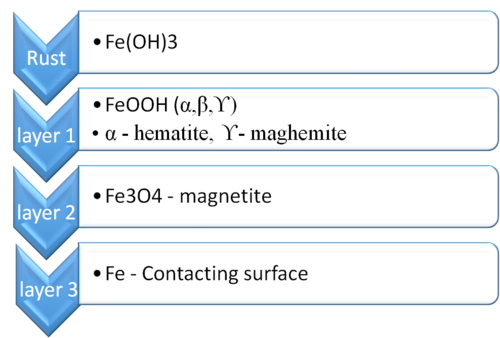
Lets consider corrosion databank now.
As you can see in a typical corrosion example, where rust i.e iron oxide forms on iron surface. The layers shown in above image are representation of the scenario. Under regular circumstances when you add a corrosion rate calculation, OLI's solver will consider speciation in a such a way that the fe surface is taken into consideration. But when you have to create a stability diagram, you need to include the CEMSE databank. Because then the time factor comes into picture. When CEMSE databank is turned on, it means the the process will not have any iron oxides by itself. But given a number of years under right circumstances the well developed rust could form along with Fe2O3 ( hematite).