Corrosion FAQ's
Frequently Asked Questions about OLI and Corrosion
Databanks
Phenomena
Standards
Comparison with ISO15156
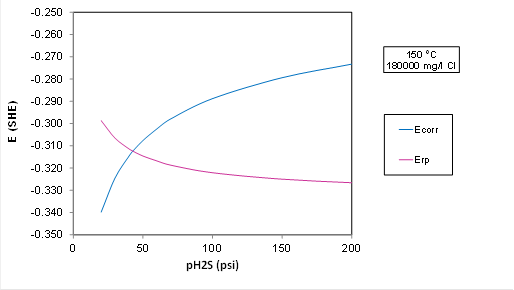
The environmental limits for the alloys in ISO15156-3 are based on experimental data with chloride concentration up to 180000 mg/l using NaCl.
A plot for Ecorr and Erp at 150 C and 5.7 m NaCl (180000 mg Cl/l) with varying partial pressure of H2S using OLI:
Plot Analysis: The transition to localized corrosion is predicted at about 50 psi H2S in OLI's predictive model, whereas ISO 15156 predicts that the alloy is OK up to 200 psi H2S. So, the electrochemical model is more conservative than the standard. But the transition would move to a substantially higher P(H2S) if we lowered the chloride concentration (because Erp strongly depends on Cl). The driving force for localized corrosion, i.e., the difference between Ecorr and Erp in the plot above is small, i.e., it is only about 50 mV at 200 psi H2S, which is within the experimental uncertainty.