Difference between revisions of "Development of the Hydronium Ion for MSE"
| Line 18: | Line 18: | ||
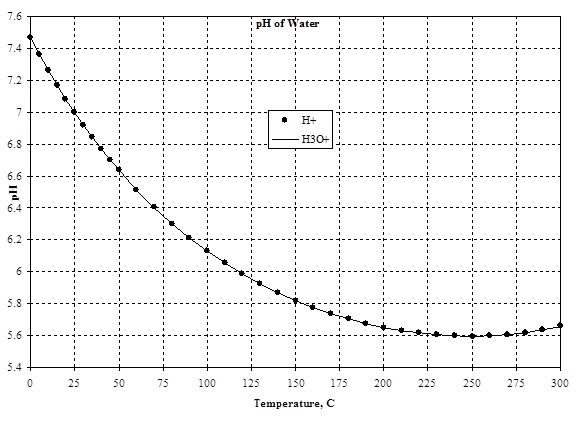
The attached figure compares the predicted pH for water using the H<sup>+</sup> and H<sub>3</sub>O<sup>+</sup> ions. The largest difference is about 0.001 pH units. | The attached figure compares the predicted pH for water using the H<sup>+</sup> and H<sub>3</sub>O<sup>+</sup> ions. The largest difference is about 0.001 pH units. | ||
| + | |||
| + | |||
| + | [[File:Hydronium image.jpg]] | ||
Latest revision as of 10:04, 15 July 2016
Hydronium Ion (H3O+)
The hydrogen ion (H+) has been replaced by the hydronium ion (H3O+) in MSEPUB. The major reason for doing this is to improve the prediction of properties for strong acids. As a result of doing this, the ionization of water is changed from:
H2O → H+ + OH-
To:
2H2O → H3O+ + OH-
The net result of these two equations is:
H3O+ = H2O + H+
In order to keep predictions the same for dilute solutions it is necessary for ![]() of this reaction to be equal to 0. Since
of this reaction to be equal to 0. Since ![]() for H+ is equal to 0 at all temperatures and pressures this means that
for H+ is equal to 0 at all temperatures and pressures this means that ![]() for H3O+ has to equal
for H3O+ has to equal ![]() for H2O at all temperatures and pressures. In OLI’s thermodynamic framework this requires the development of Helgeson parameters for H3O+ by matching
for H2O at all temperatures and pressures. In OLI’s thermodynamic framework this requires the development of Helgeson parameters for H3O+ by matching ![]() for H2O as closely as possible. This was done for a temperature range of 0 to 300
for H2O as closely as possible. This was done for a temperature range of 0 to 300 ![]() C and for pressures up to over 1000 atmospheres.
C and for pressures up to over 1000 atmospheres.
The attached figure compares the predicted pH for water using the H+ and H3O+ ions. The largest difference is about 0.001 pH units.