Difference between revisions of "How to choose a subsystem"
| (4 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
Please note: | Please note: | ||
| − | What is a redox subsystem? | + | '''What is a redox subsystem?''' |
* A set of species that contain a given element in all possible oxidation | * A set of species that contain a given element in all possible oxidation | ||
| Line 17: | Line 17: | ||
Now let us study an example: | Now let us study an example: | ||
| − | + | A stability diagram calculation with Stainless Steel 304 as contact surface will look like this: | |
| − | + | [[File:All the subsystems.png|800px]] | |
| + | |||
| + | |||
| + | Where we have iron, nickel and chromium , all three present in the same system. | ||
[[File:Spec subsystem.png]] | [[File:Spec subsystem.png]] | ||
| − | |||
| − | [[File:Fe subsystem 1.png]] | + | == Iron subsystem == |
| + | |||
| + | |||
| + | The iron subsystem will look as follows: | ||
| + | |||
| + | Please note that both FeCr2O4 and NiFe2O4 will be shown to be present. | ||
| + | |||
| + | [[File:Fe subsystem 1 eliminated.png|800px]] | ||
| + | |||
| + | |||
| + | == Nickel subsystem == | ||
| + | |||
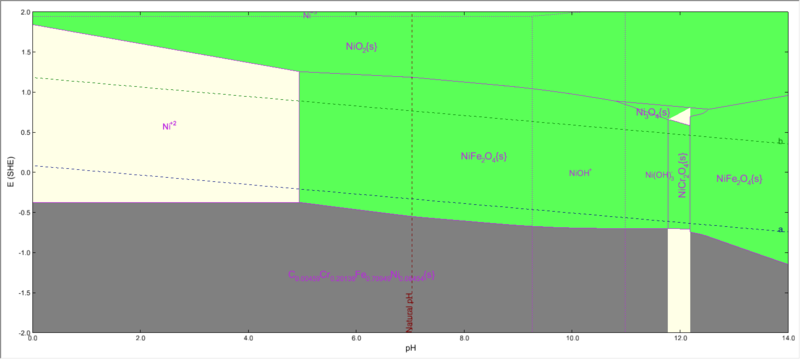
| + | When we select the nickel subsystem from the specs options, you will see that NiFe2O4 is present. | ||
| + | |||
| + | [[File:Ni subsystem eliminated.png|800px]] | ||
| − | + | == Chromium subsystem == | |
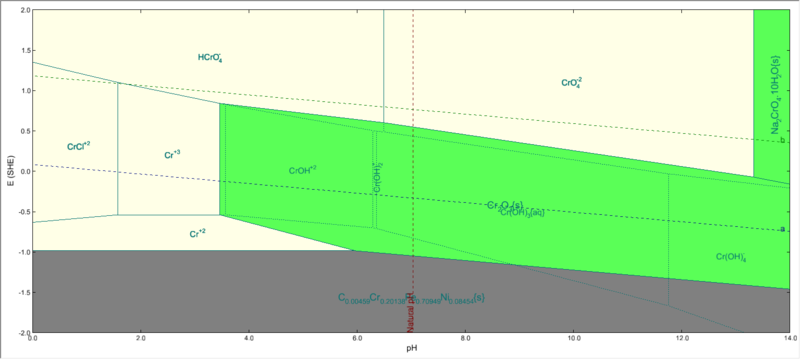
| − | + | But when you highlight chromium, only Cr2O3 will be shown as stable: | |
| − | + | [[File:Cr subsystem eliminated.png|800px]] | |
| − | |||
| − | The reason being that, certain species(like FeCr2O4) will be most stable at a certain E & pH among all Fe-containing species. But this species is not the most stable among all Cr-containing species. ( In other words , it is metastable with respect to a species that does not contain Fe). | + | The reason for only Cr2O3 being stable is that, certain species(like FeCr2O4) will be most stable at a certain E & pH among all Fe-containing species. But this species is not the most stable among all Cr-containing species. ( In other words , it is metastable with respect to a species that does not contain Fe). |
[[Category: Corrosion]] | [[Category: Corrosion]] | ||
Latest revision as of 12:21, 12 August 2015
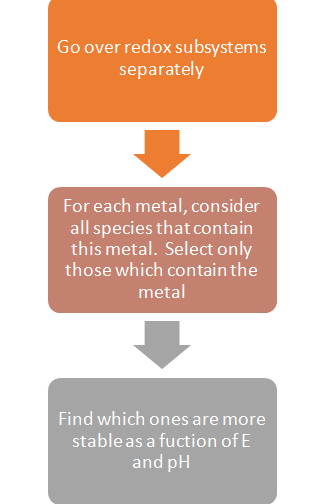
The common question faced by users while generating a stability diagram is: How to choose a subsystem and how to determine the stability of metal containing species in certain subsystems?
The logical approach to take for this is the following algorithm:
Please note:
What is a redox subsystem?
- A set of species that contain a given element in all possible oxidation
states.
- Example: The iron subsystem consists of all species that contain Fe0, Fe2+ and Fe3+.
Now let us study an example:
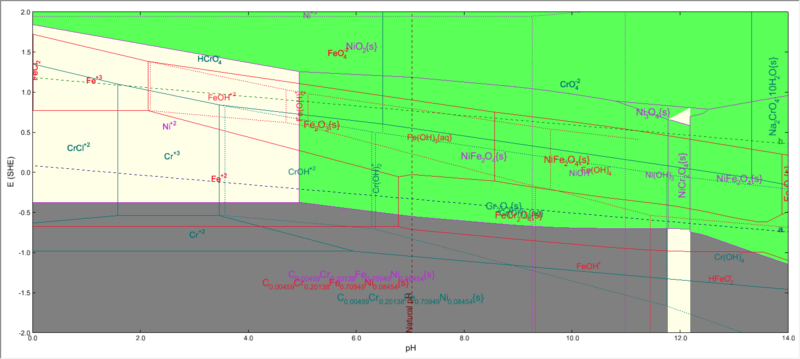
A stability diagram calculation with Stainless Steel 304 as contact surface will look like this:
Where we have iron, nickel and chromium , all three present in the same system.
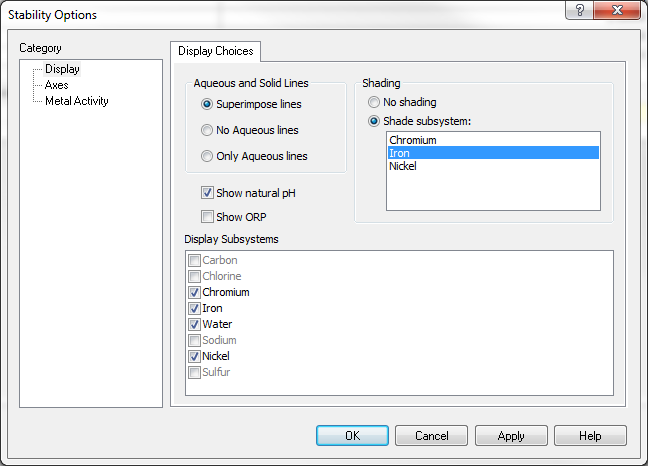
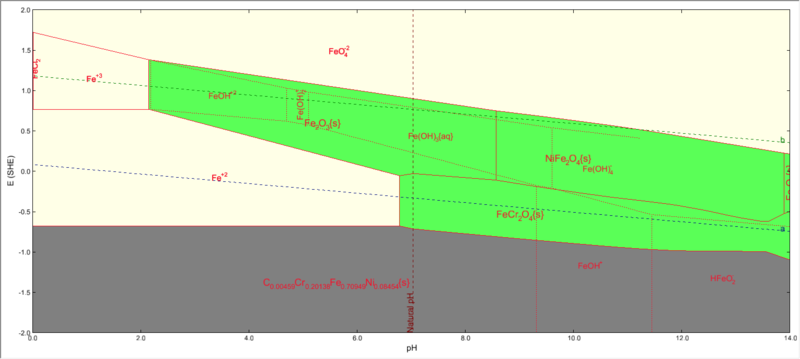
Iron subsystem
The iron subsystem will look as follows:
Please note that both FeCr2O4 and NiFe2O4 will be shown to be present.
Nickel subsystem
When we select the nickel subsystem from the specs options, you will see that NiFe2O4 is present.
Chromium subsystem
But when you highlight chromium, only Cr2O3 will be shown as stable:
The reason for only Cr2O3 being stable is that, certain species(like FeCr2O4) will be most stable at a certain E & pH among all Fe-containing species. But this species is not the most stable among all Cr-containing species. ( In other words , it is metastable with respect to a species that does not contain Fe).