Difference between revisions of "How to choose a subsystem"
| Line 4: | Line 4: | ||
[[File:Algorithm.png]] | [[File:Algorithm.png]] | ||
| + | |||
| + | |||
| + | Please note: | ||
| + | |||
| + | What is a redox subsystem? | ||
| + | |||
| + | * A set of species that contain a given element in all possible oxidation | ||
| + | states. | ||
| + | * Example: The iron subsystem consists of all species that contain Fe0, Fe2+ and Fe3+. | ||
Revision as of 11:41, 12 August 2015
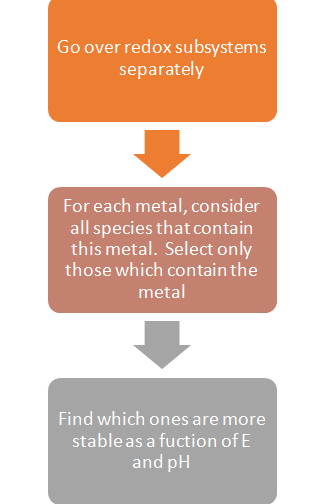
The common question faced by users while generating a stability diagram is: How to choose a subsystem and how to determine the stability of metal containing species in certain subsystems?
The logical approach to take for this is the following algorithm:
Please note:
What is a redox subsystem?
- A set of species that contain a given element in all possible oxidation
states.
- Example: The iron subsystem consists of all species that contain Fe0, Fe2+ and Fe3+.