Difference between revisions of "How to choose a subsystem"
| Line 17: | Line 17: | ||
Now let us study an example: | Now let us study an example: | ||
| − | + | A stability diagram calculation with Stainless Steel 304 as contact surface will look like this: | |
| + | |||
| + | [[File:All the subsystems.png]] | ||
| + | |||
| + | |||
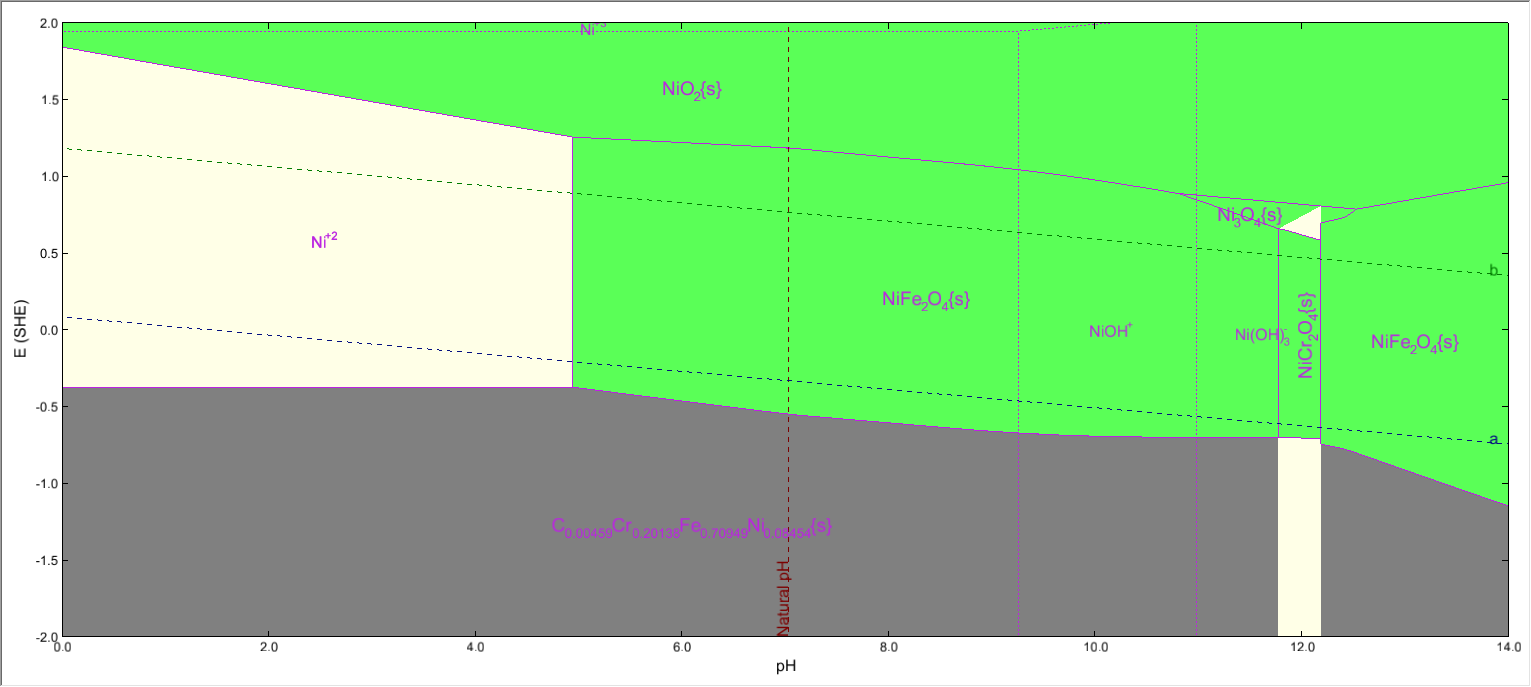
| + | Where we have Iron, Nickel and Chromium , all three present in the same system. | ||
When we highlight the Iron subsystem, both FeCr2O4 and NiFe2O4 will be shown to be present. | When we highlight the Iron subsystem, both FeCr2O4 and NiFe2O4 will be shown to be present. | ||
| Line 25: | Line 30: | ||
The iron subsystem will look at follows: | The iron subsystem will look at follows: | ||
| − | [[File:Fe subsystem 1.png]] | + | [[File:Fe subsystem 1 eliminated.png]] |
When we select the Nickel subsystem from the specs options, you will see that NiFe2O4 is present. | When we select the Nickel subsystem from the specs options, you will see that NiFe2O4 is present. | ||
| − | [[File:Ni subsystem.png]] | + | [[File:Ni subsystem eliminated.png]] |
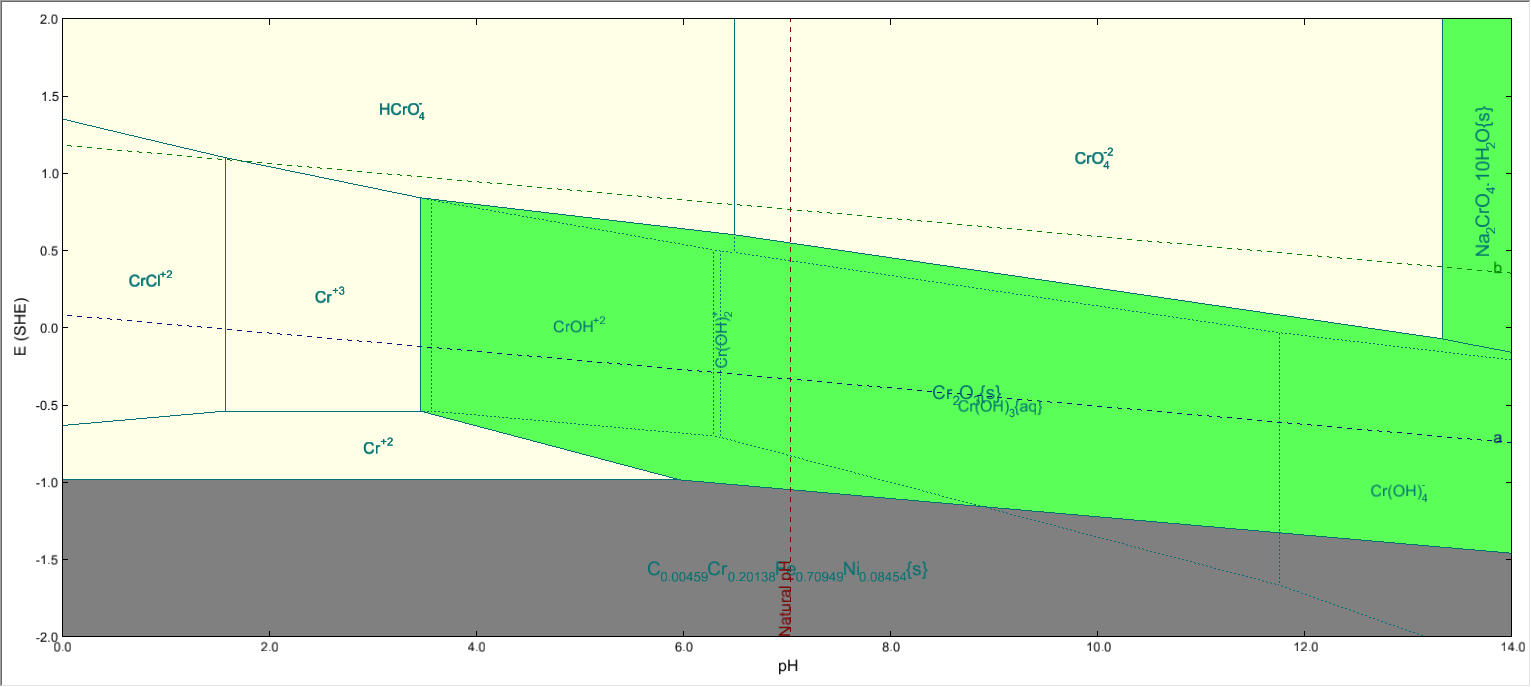
But when you highlight Chromium, only Cr2O3 will be shown as stable: | But when you highlight Chromium, only Cr2O3 will be shown as stable: | ||
| − | [[File:Cr subsystem.png]] | + | [[File:Cr subsystem eliminated.png]] |
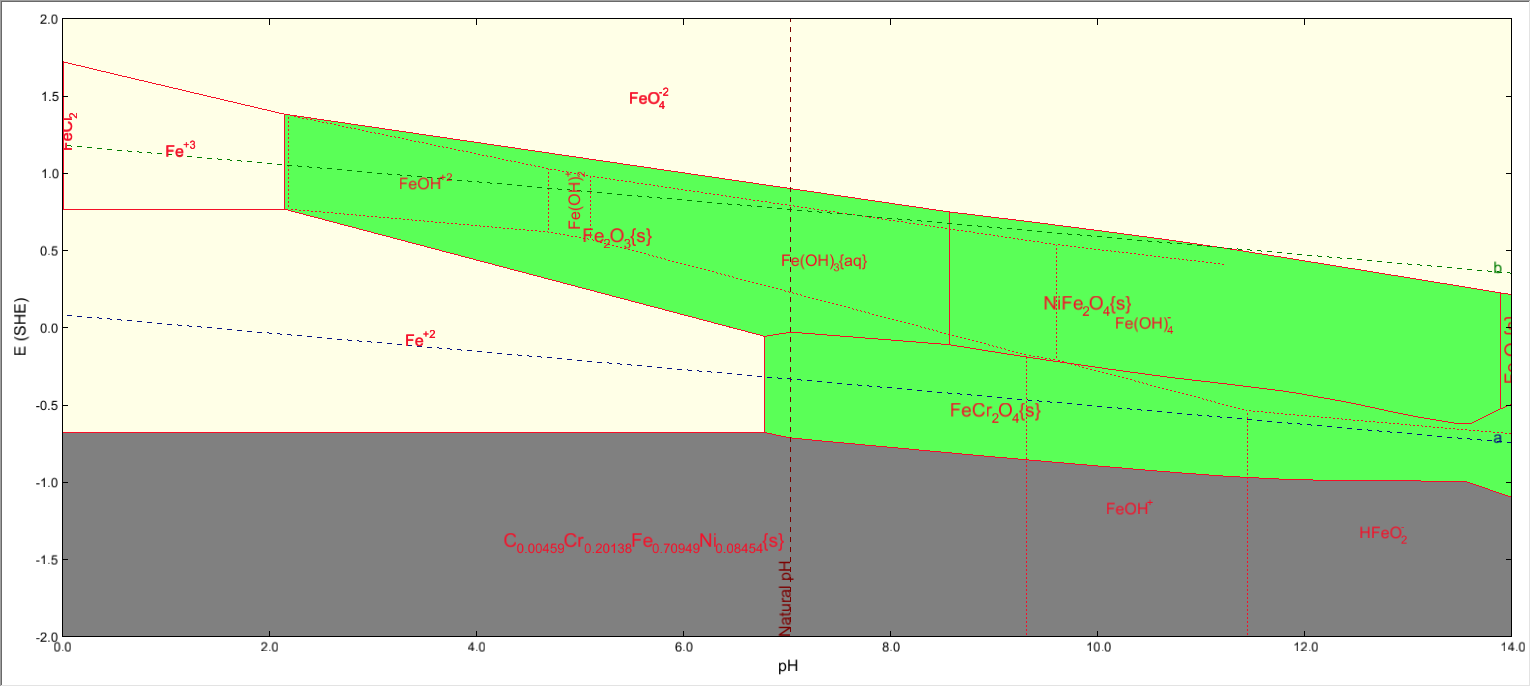
The reason being that, certain species(like FeCr2O4) will be most stable at a certain E & pH among all Fe-containing species. But this species is not the most stable among all Cr-containing species. ( In other words , it is metastable with respect to a species that does not contain Fe). | The reason being that, certain species(like FeCr2O4) will be most stable at a certain E & pH among all Fe-containing species. But this species is not the most stable among all Cr-containing species. ( In other words , it is metastable with respect to a species that does not contain Fe). | ||
[[Category: Corrosion]] | [[Category: Corrosion]] | ||
Revision as of 12:02, 12 August 2015
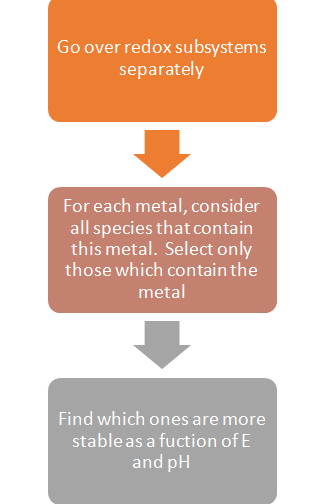
The common question faced by users while generating a stability diagram is: How to choose a subsystem and how to determine the stability of metal containing species in certain subsystems?
The logical approach to take for this is the following algorithm:
Please note:
What is a redox subsystem?
- A set of species that contain a given element in all possible oxidation
states.
- Example: The iron subsystem consists of all species that contain Fe0, Fe2+ and Fe3+.
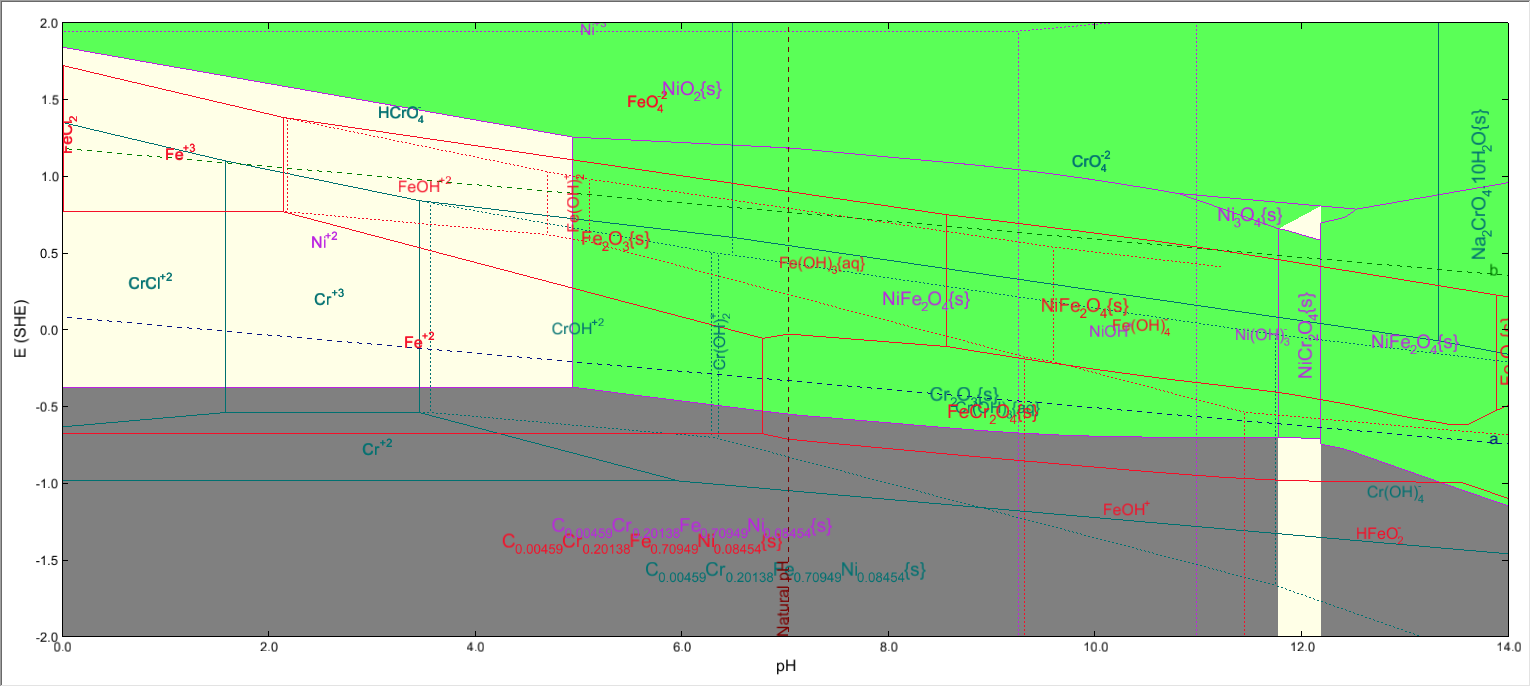
Now let us study an example:
A stability diagram calculation with Stainless Steel 304 as contact surface will look like this:
Where we have Iron, Nickel and Chromium , all three present in the same system.
When we highlight the Iron subsystem, both FeCr2O4 and NiFe2O4 will be shown to be present.
The iron subsystem will look at follows:
When we select the Nickel subsystem from the specs options, you will see that NiFe2O4 is present.
But when you highlight Chromium, only Cr2O3 will be shown as stable:
The reason being that, certain species(like FeCr2O4) will be most stable at a certain E & pH among all Fe-containing species. But this species is not the most stable among all Cr-containing species. ( In other words , it is metastable with respect to a species that does not contain Fe).