Difference between revisions of "Modeling the Chemistry of Carbon Dioxide - Rich Phases with Impurities"
| Line 11: | Line 11: | ||
*: - S<sup>o</sup> – H<sub>2</sub>O | *: - S<sup>o</sup> – H<sub>2</sub>O | ||
*:: Solubility of S<sup>o</sup> in H<sub>2</sub>O is important if an aqueous phase forms | *:: Solubility of S<sup>o</sup> in H<sub>2</sub>O is important if an aqueous phase forms | ||
| − | *:: Model development requires considering the | + | *:: Model development requires considering the S<sup>o</sup> – H<sub>2</sub>O system because the reference state for liquid-phase species is infinite dilution in water |
*: - S<sup>o</sup> – CO<sub>2</sub> | *: - S<sup>o</sup> – CO<sub>2</sub> | ||
*:: Solubility of S<sup>o</sup> in CO<sub>2</sub> | *:: Solubility of S<sup>o</sup> in CO<sub>2</sub> | ||
| Line 25: | Line 25: | ||
*: - In the pure liquid and solution phase, S<sup>o</sup><sub>8</sub> predominates | *: - In the pure liquid and solution phase, S<sup>o</sup><sub>8</sub> predominates | ||
*: - In the CO<sub>2</sub> phase, solvated S<sup>o</sup>-CO<sub>2</sub> species may appear | *: - In the CO<sub>2</sub> phase, solvated S<sup>o</sup>-CO<sub>2</sub> species may appear | ||
| + | |||
| + | |||
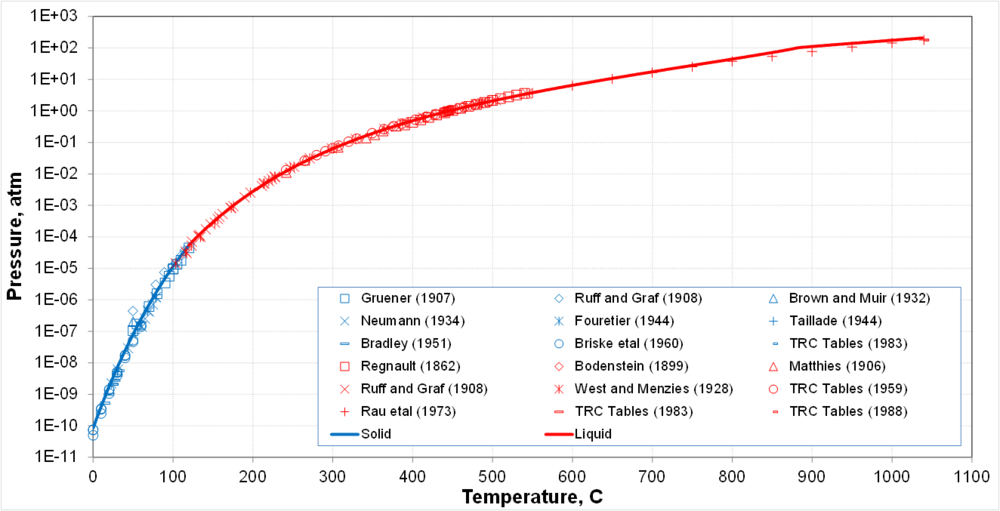
| + | '''Vapor pressure of S<sup>o</sup>'''<br> | ||
| + | [[File:01.png | 1000px |boarder]] | ||
| + | |||
| + | * Solid-vapor equilibrium transitions into liquid-vapor equilibrium at the triple point | ||
| + | * Calculations are supported by a large body of generally consistent data | ||
Revision as of 09:57, 4 November 2016
Dense Phase CO2 Corrosion: Modeling the Chemistry of CO2 – Rich Phases with Impurities
- Objectives:
- - Model the solubility of So in CO2 - rich phases in order to predict whether solid So can drop out in transmission lines
- - Predict whether So can undergo reactions in the presence of water
- Chemical subsystems to be modeled
- - Pure So
- Volatility of pure So provides a baseline for its solubility in gas phase
- - So – H2O
- Solubility of So in H2O is important if an aqueous phase forms
- Model development requires considering the So – H2O system because the reference state for liquid-phase species is infinite dilution in water
- - So – CO2
- Solubility of So in CO2
- - Redox reactions of sulfur in aqueous media
- Will be important for future modeling of reactions of SOx and NOx
- - Pure So
- Polymeric species of sulfur
- - Numerous sulfur species up to S20 have been detected
- - In the gas phase, eight multimers (So1 through So8) have been assumed in the model
- Thermochemical data are well established for So1 through So8
- So8 is dominant at normal and moderate conditions
- Lower multimers become prevalent at higher temperatures
- - In the pure liquid and solution phase, So8 predominates
- - In the CO2 phase, solvated So-CO2 species may appear
- Solid-vapor equilibrium transitions into liquid-vapor equilibrium at the triple point
- Calculations are supported by a large body of generally consistent data