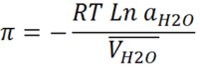Difference between revisions of "Osmotic Pressure"
| Line 8: | Line 8: | ||
| − | |||
Where | Where | ||
| − | π is the osmotic pressure | + | π is the osmotic pressure |
| − | R is the gas constant | + | R is the gas constant |
| − | T is the temperature | + | T is the temperature |
| − | aH2O is the activity of water at temperature T | + | aH2O is the activity of water at temperature T |
| − | + | VH2O is the partial molal volume of water at temperature T | |
Revision as of 08:44, 25 September 2014
OLI is asked frequently how is the osmotic pressure calculated in the software.
We use a very traditional method of callculating the osmotic pressure.
Where
π is the osmotic pressure
R is the gas constant
T is the temperature
aH2O is the activity of water at temperature T
VH2O is the partial molal volume of water at temperature T