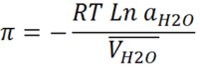Difference between revisions of "Osmotic Pressure"
| Line 19: | Line 19: | ||
VH2O is the partial molal volume of water at temperature T | VH2O is the partial molal volume of water at temperature T | ||
| + | |||
| + | [[Category:Tips]] | ||
Latest revision as of 08:48, 25 September 2014
OLI is asked frequently how is the osmotic pressure calculated in the software.
We use a very traditional method of callculating the osmotic pressure.
Where
π is the osmotic pressure
R is the gas constant
T is the temperature
aH2O is the activity of water at temperature T
VH2O is the partial molal volume of water at temperature T