Difference between revisions of "Simulating evaporation"
(Created page with " == Evaporation in a closed system == A liquid in a closed system will not start to evaporate until its boiling point is reached. Further evaporation will occur when the tem...") |
|||
| Line 8: | Line 8: | ||
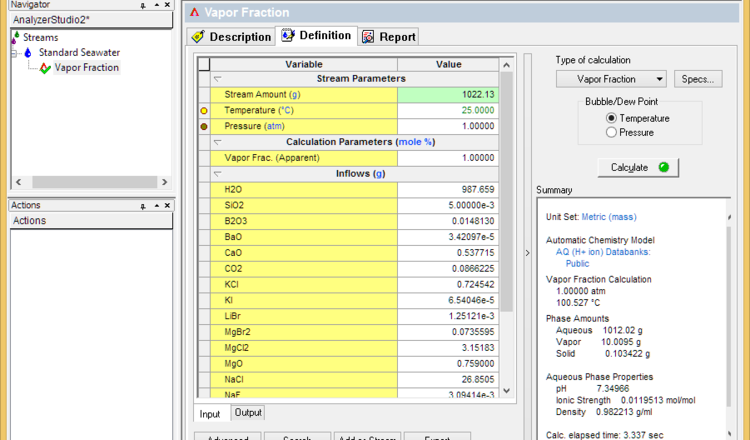
* Set the vapor fraction to 1% and calculate. This will determine the initial boiling point | * Set the vapor fraction to 1% and calculate. This will determine the initial boiling point | ||
| − | [[File:VaporFractionSeawater-1pct.png]] | + | [[File:VaporFractionSeawater-1pct.png|750px]] |
| Line 14: | Line 14: | ||
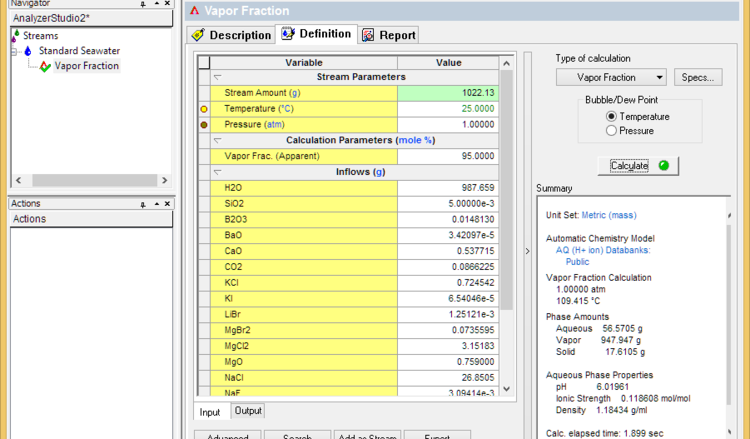
* Set the vapor fraction to 99% less the % of dissolved solids (seawater for example contains about 3.6% solids). If the calculation fails, then lower the vapor fraction until it converges. This sets the dryness temperature | * Set the vapor fraction to 99% less the % of dissolved solids (seawater for example contains about 3.6% solids). If the calculation fails, then lower the vapor fraction until it converges. This sets the dryness temperature | ||
| − | [[File:VaporFractionSeawater-95pct.png]] | + | [[File:VaporFractionSeawater-95pct.png|750px]] |
| Line 20: | Line 20: | ||
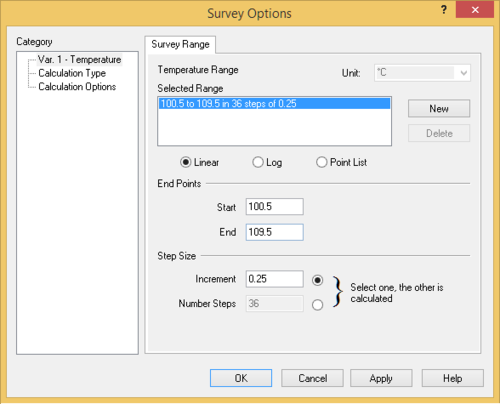
* Add a Survey calculation - Use the default Temperature survey | * Add a Survey calculation - Use the default Temperature survey | ||
* Set the initial and final temperatures to the values obtained above. Add sufficient increments (e.g., 10-20) so that the plot has good resolution. | * Set the initial and final temperatures to the values obtained above. Add sufficient increments (e.g., 10-20) so that the plot has good resolution. | ||
| + | |||
| + | [[File:SeawaterEvap-TempertureSurveySpecScreen.png|500px]] | ||
| + | |||
* Plot the liquid volume vs. temperature first. See if sufficient liquid evaporated for your case. | * Plot the liquid volume vs. temperature first. See if sufficient liquid evaporated for your case. | ||
Revision as of 12:40, 30 July 2014
Evaporation in a closed system
A liquid in a closed system will not start to evaporate until its boiling point is reached. Further evaporation will occur when the temperature increases. Therefore, the fastest way to simulate evaporation is using a Temperature survey. We will use Standard Seawater to present this method.
- Add a single point calculation
- Change the calculation type to Vapor Fraction
- Set the vapor fraction to 1% and calculate. This will determine the initial boiling point
- Set the vapor fraction to 99% less the % of dissolved solids (seawater for example contains about 3.6% solids). If the calculation fails, then lower the vapor fraction until it converges. This sets the dryness temperature
- Add a Survey calculation - Use the default Temperature survey
- Set the initial and final temperatures to the values obtained above. Add sufficient increments (e.g., 10-20) so that the plot has good resolution.
- Plot the liquid volume vs. temperature first. See if sufficient liquid evaporated for your case.