When to use corrosion and geochemical databanks
When to use Corrosion and Geochemical databank
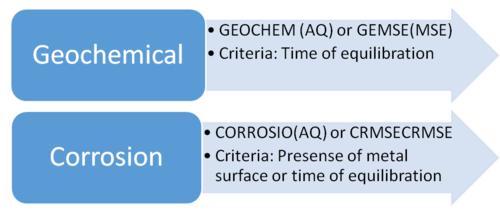
When you use OLI software, there are certain calculations that need additional databanks to be included for specific predictions. The main special databanks are geochemical (i.e. GEOCHEM in the AQ model and GEMSE in the MSE model) and corrosion-related (i.e., CORROSIO in the AQ model and CRMSE in the MSE model).
There is a fine difference between scenarios which govern whether the databanks should be included or not. One of the key factors is the time of equilibration. Would a particular species be formed under process conditions? Or can it only be formed over a significant period of time, for example a geological timeframe?
For the geochemical databanks (GEOCHEM and GEMSE), the controlling parameter is only the equilibration time. If a component forms only over a geological time scale, then it is necessary to include one of the geochemical databanks. Please remember that under chemical process circumstances this solid would not realistically form and would not be predicted as a part of the system.
A good example for this is the SiO2 system. OLI extensively worked on this system. Now SiO2 and its two forms
-> SiO2 ( Amorphous)
-> SiO2 ( Quartz)
are included in MSE and GEMSE databanks respectively. Now under chemical process conditions, one would expect the amorphous silicon dioxide to be present in a system. But when a geological timescale (or a special crystal growth process) is involved, then it becomes necessary to include the quartz structure, and then the geochemical databank comes into picture.
Lets consider corrosion databank now.
Under typical process conditions, Fe(OH)3 can potentially form. This is why we have Fe(OH)3 in the Public and MSEPUB databanks.
The other oxides/hydroxides may form under two scenarios. First, they may form on a corroding surface. For example, the passive film on iron is typically composed of an inner layer of Fe3O4 and an outer layer of FeOOH. Therefore, we need to include these oxides in corrosion calculations. This is why they are incorporated into CORROSIO and CRMSE. The second scenario is aging. For example, hematite (Fe2O3) may result from the aging of Fe(OH)3 either on the surface or in the bulk. To simulate such a scenario, we also need the corrosion databanks.
For a list of components in the Corrosion databank , please follow the following link: