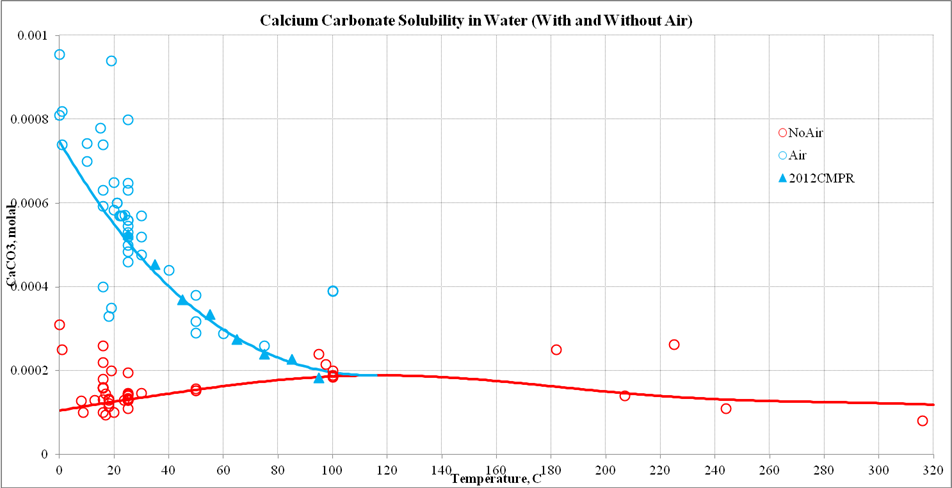
Calcium Carbonate Solubility
The solubility of calcite (CaCO3, Calcite) seems to show an inverse solubility with respect to temperature. This seems contradictory to what is normally observed in the field.
The confusion occurs because of the assumed presence of carbon dioxide (CO2). Normally atmospheric air contains approximately 300 ppm CO2. However, when initially using the OLI software such as the OLI Studio program, the user often forgets to add the CO2 to the calculation.
Here is a plot of CaCO3 solubility versus temperature with and without CO2:
In the case of no air, there is a slight increase in the solubility of CaCO3 with respect to temperature although there is significant scatter to the data. This is what is reported.
With atmospheric CO2 present, the decrease in solubility is quite pronounced. A recent paper in 2012 also shows this trend. In some earlier experiments, the amount of atmospheric CO2 was not recorded which gave the decrease in solubility which we all expected.
References:
Air
1846
Fresenius R., "Ueber die Loslichkeitsverhaltnisse von Einigen, bei der Quantitativen Analyse als Bestimmungsformen etc. Dienenden Niederschlagen", Justus Liebigs Annalen der Chemie, 59, (1), 117-128, 1846.
1852
Kremers P., "Ueber den Zusammenhang des Specifischen Gewichtes Chemischer Verbindungen Mit Ihrer Aufloslichkeit in Wasser, Nebst Einer Daraus Abgeleiteten Theorie der Wahlverwandtschaften", Annalen der Physik, 161, (2), 246-262, 1852.
1901
Lehmann K. B., Die Methoden der Praktischen Hygiene Wiesbaden, 218, 1901.
1902
Cameron F. K., Seidell A., "Solubility of Calcium Carbonate in Aqueous Solutions of Certain Electrolytes in Equilibrium with Atmospheric Air", Journal of Physical Chemistry, 6, (1), 50-56, 1902.
1912
Kendall J., "The Solubility of Calcium Carbonate in Water", Philosophical Magazine, 23, (6), 958-976, 1912.
1915
Johnston J., "The Solubility - Product Constant of Calcium and Magnesium Carbonates", Journal of the American Chemical Society, 37, (9), 2001-2020, 1915.
1915
Wells R. C., "The Solubility of Calcite in Water in Contact With the Atmosphere, and Its Variation With Temperature", Journal of the Washington Academy of Sciences, 5, 617-622, 1915.
1929
Frear G. L., Johnston J., "The Solubility of Calcium Carbonate (Calcite) in Certain Aqueous Solutions at 25 C", Journal of the American Chemical Society, 51, (7), 2082-2093, 1929.
1929
Heyrovsky J., Berezicky S., Collection of Czechoslovak Chemical Communications, 1, 19-46, 1929.
1929
Kline W. D., "Experimental Redeterminations of the Solubility of Calcite in Water at 25 C", Journal of the American Chemical Society, 51, (7), 2086-2086, 1929.
1932
Leick J., "The Solubilities of Calcium Carbonate and of Magnesium Carbonate in Water That is Free From Carbonic Acid", Zeitschrift fur Analytische Chemie, 87, 415-422, 1932.
1933
Leick J., "The Equilibria in the Conversion of Sodium Carbonate, Sodium Hydroxide, Calcium Hydroxide and Trisodium Phosphate with Calcium- and Magnesium Sulphate", Zeitschrift fur Anorganische und Allgemeine Chemie, 210, (2), 203-209, 1933.
1937
Wattenberg V. H., Timmermann E., "Die Loslichkeit von Magnesiumkarbonat und Strontiumkarbonat in Seewasser", Kieler Meeresforschungen, 11, (1), 81-94, 1937.
1952
Shternina E. B., Frolova E. V., "The Solubility of Calcite in the Presence of CO2 and NaCl", Izvestiya Sektora Fiziko Khimicheskogo Analiza Institut Obshchei Neorganicheskoi Khimii Akademiia Nauk SSSR, 21, 271-287, 1952.
2004
Loos D., Pasel C., Luckas M., Schmidt K. G., Herbell J. D., "Experimental Investigation and Modelling of the Solubility of Calcite and Gypsum in Aqueous Systems at Higher Ionic Strength", Fluid Phase Equilibria, 219, (2), 219-229, 2004.
No Air
1857
Bineau A., "Remarques sur les Dissolutions de Quelques Carbonates et Notamment du Carbonate de Chaux", Annales de Chimie et de Physique, 51, (3), 290-305, 1857.
1872
Schloesing T., "Sur la Dissolution du Carbonate de Chaux par L'Acide Carbonique", Comptes Rendus Hebdomadaires des Seances de L'Academie des Sciences, 75, 70-73, 1872.
1892
Lubavin N. N., Journal of the Russian Physical Chemical Society, 24, 389, 1892.
1893
Holleman A. F., Kohlrausch F., Rose F., "Bestimmungen der Loslichkeit Sogenannter Unloslicher Salze", Zeitschrift fur Physikalische Chemie Stochiometrie und Verwandtschaftslehre, 12, 125-139, 1893.
1906
LeBlanc M., Novotny K., "The Caustization of Sodium Carbonate and Kalium Carbonate With Calk", Zeitschrift fur Anorganische Chemie, 51, (1), 181-201, 1906.
1907
Cameron F. K., Robinson W. O., "The Solubility of Calcium Carbonate in Aqueous Solutions of Potassium Chloride and Potassium Sulphate at 25 C", Journal of Physical Chemistry, 11, (8), 577-580, 1907.
1911
Prudhomme M., Journal de Chimie Physique et de Physico Chimie Biologique, 9, 532, 1911.
1915
Johnston J., "The Solubility - Product Constant of Calcium and Magnesium Carbonates", Journal of the American Chemical Society, 37, (9), 2001-2020, 1915.
Wells R. C., "The Solubility of Calcite in Water in Contact With the Atmosphere, and Its Variation With Temperature", Journal of the Washington Academy of Sciences, 5, 617-622, 1915.
1923
Askew H. O., "Solubility and Hydrolysis of Calcium Carbonate", Transactions and Proceedings of the Royal Society of New Zealand, 54, 791-796, 1923.
1925
Pratolongo U., Atti Della Accademia Nazionale dei Lincei, 1, (6), 238-243, 1925.
1925
Stumper R., Bulletin des Societes Chimiques Belges, 34, 422-427, 1925.
1929
Pauli W., Stenzinger T., "On Solubility Influence of Not Easily Dissoluble Lime Salts Through Protein Borders and On Carbonic Acid Compounds of Proteins", Biochemische Zeitschrift, 205, 71-103, 1929.
1931
Dubrisay R., Francois R., "Solubilite du Carbonate de Calcium Dans L'Eau en Presence de Chlorures Alcalins", Comptes Rendus Hebdomadaires des Seances de L'Academie des Sciences, 192, 741-743, 1931.
1932
Bar O., "Beitrag zum Thema Dolomitentstehung", Centralblatt fur Mineralogie Geologie und Palaontologie, 46-62, 1932.
1932
Straub F. G., "Solubility of Calcium Sulfate and Calcium Carbonate at Temperatures Between 182 and 316 C", Industrial and Engineering Chemistry, 24, (8), 914-917, 1932.
1938
Franquin J., Marecaux P., 18th Congress Chim. Industrielle, 532c-547c, 1938.
1944
Wesley W., Chemiker Zeitung, 68, 189, 1944.
1963
Malinin S. D., "Experimental Investigation of Calcite and Witherite Solubility Under Hydrothermal Conditions", Geochemistry, (7), 650-667, 1963.
2004
Loos D., Pasel C., Luckas M., Schmidt K. G., Herbell J. D., "Experimental Investigation and Modelling of the Solubility of Calcite and Gypsum in Aqueous Systems at Higher Ionic Strength", Fluid Phase Equilibria, 219, (2), 219-229, 2004.
2012CMPR
Coto B., Martos C., Pena J. L., Rodriguez R., Pastor G., "Effects in the Solubility of CaCO3: Experimental Study and Model Description", Fluid Phase Equilibria, 324, 1-7, 2012.