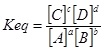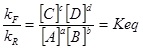ESP Examples: Using Constrained Reaction Kinetics
Overview
Frequently a user wants to describe a chemical reaction in terms of reaction kinetics rather than equilibrium. The user may also want to constrain the reaction kinetics such that the forward and reverse rates of reaction do not exceed the limits placed on it by chemical equilibrium.
To briefly explain the procedure we first need to look at a generic equilibrium reaction:
The standard equilibrium constant expression is3 :
1This manual is for version 6.7 of OLI/Engine.
2You can click these links for the reference material.
3We are ignoring activity coefficients to simplify the example.
At equilibrium the forward rate and the reverse rate are equal.
This expands to:
Upon re-arrangement, we get
Example 1: Standard Reaction Kinetics
The files for this example can be found in compressed format by following this link:
In this example we are using standard reaction kinetics to hydrolyze ammonia. The overall reaction is:
We know the forward rate constant (and hence the forward reaction rate) but we wish to constrain the forward and reverse reaction rates to the thermodynamic equilibrium constant stored in the OLI Databases.
To do this we create a standard model file and add the following section:
KINETICS REAC1 NH3AQ+H2O=NH4ION+OHION RATE1 STD AF=3.0 BF=0 KR=KF/KEQ ER1=1.0 ER2=1.0 EP1=1.0 EP2=1.0
This section is added to the end of the model file (MOD) but before the END statement. A special note: The standard equilibrium equation in the EQUILIBRIUM section must remain so we can obtain the equilibrium constant. In non-constrained reaction kinetics we would be forced to remove the default equilibrium equation.
The standard reaction rate syntax applies here with the addition of a new statement.
KR=KF/KEQ
This forces the reverse rate constant to be constrained by the equilibrium constant KEQ. In this example, the forward rate constant is being defined via the Arrehnius equation:
Example 2: Non-Standard Reaction Kinetics4
The files for this example can be found in compressed format by following this link:
In this example we are using non-standard reaction kinetics to hydrolyze ammonia. The overall reaction is:
We know the forward rate constant (and hence the forward reaction rate) but we wish to constrain the forward and reverse reaction rates to the thermodynamic equilibrium constant stored in the OLI Databases.
To do this we create a standard model file and add the following section:
KINETICS
REAC1 NH3AQ+H2O=NH4ION+OHION
RATE1 SPEC
DEFINE FXRATE=LNH3AQ+ANH3AQ+LH2O+AH2O
DEFINE RXRATE=LNH4ION+ANH4ION+LOHION+AOHION
DEFINE KF1=3
DEFINE KR1=KF1/KEQ1
DEFINE RATE1=(KF1*EXP(FXRATE)-KR1*EXP(RXRATE))*VOLLIQ/1000.
This section is added to the end of the model file (MOD) but before the END statement. A special note: The standard equilibrium equation in the EQUILIBRIUM section must remain so we can obtain the equilibrium constant. In non-constrained reaction kinetics we would be forced to remove the default equilibrium equation.
The non-standard reaction rate syntax applies here with the addition of a new statement. In this case we may have several reaction rates and we need to create specific variables tied to the reaction rates. Here we have appended the number “1” to denote that these variables are linked to REAC1
KR1=KF1/KEQ1
This forces the reverse rate constant to be constrained by the equilibrium constant KEQ.
4It is beyond the scope of this document to explain how to use non-standard reaction kinetics.
This was former Tip75