HF Chemistry and Modeling
Hydrofluoric acid chemistry is difficult to model which is why OLI chose to use the MSE thermodynamic framework. On this page OLI has posted some issues about HF modeling.
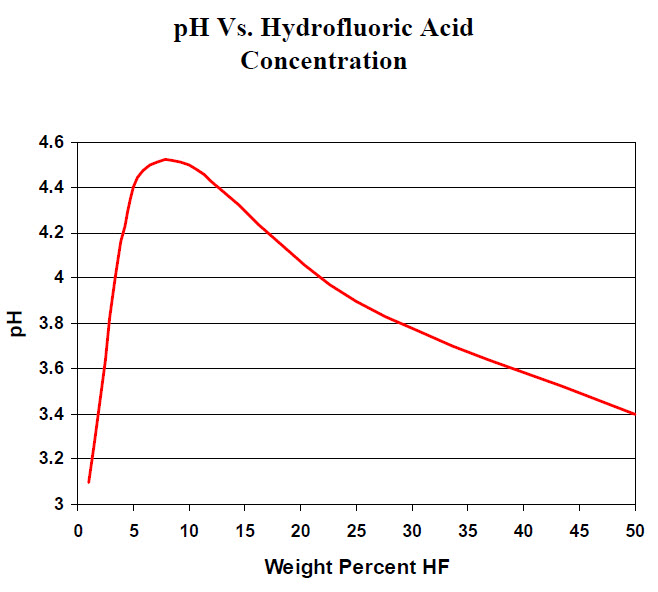
pH and HF
There have been some papers and homographs published about HF solutions in water and pH. For example, this image was obtained from a manufacturer's website:
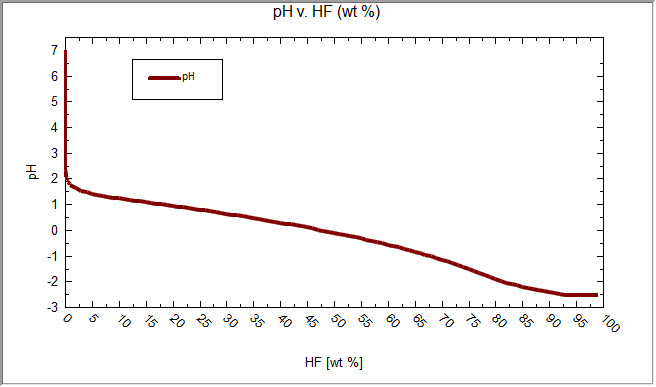
OLI is highly suspect of this prediction. The results from OLI Studio version 9.6.3 at 25oC and 1.0 Atm is as follows:
If any person knows the reason for this major difference please contact OLI Systems so we can address this issue.
Why is HF2-1 not in the list of species but H2F+1 is?
- The “speciation” type of data (relevant to the pH predictions in aqueous solutions) used in model development are the pKa values as a function of temperature (to 300C) from literature (e.g. Shock et al. 1989; Ellis (1963); Richardson and Holland (1979))
- Other relevant speciation data used are the relative (small) amount of ionic concentration in anhydrous HF from conductivity measurements (e.g. Miki et al. (1990); Kilpatrick et al (1954)
- H2F+ is the product of self-dissociation (or autoprotolysis) of anhydrous HF, this has been reported in the literature, this species has been introduced in our HF model: 2HF ⇌ H2F+ + F-
- No explicit HF2-1 was introduced. HF0 and F- are treated as separate entities rather than a complex between the two. In concentrated HF solutions (such as anhydrous HF), these two species can be in close proximity, but not as a single entity (e.g. complex)
- By introducing H2F+1, together with F- and HF0, the model yields results in good agreement with literature data, without having to introduce extra complexes such as HF2-1; this has simplified the model approach
- Other data used in modeling HF include vapor pressure (pure HF and HF + H2O), osmotic coefficients and water activities, heats of dilution, solution heat capacities, vapor phase compressibilities, and solution densities. Combined analysis of different types of data in parameterization allows the model to give reliable predictions for properties directly related to Gibbs energy (e.g. VLE) and speciation (for pH) and their temperature variations (as constrained by enthalpy and heat capacity).