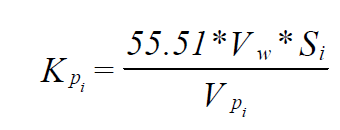Sparingly Soluble Organics
The starting point for developing of K-Values for sparingly soluble organics is the following relationship developed in the Transactions of the 11th User Conference for OLI Systems Inc.
Where:
K pi = ProChem K-Value for species i (1/atm)
Vw = Molar Volume of Water (m3/mole)
Si = Solubility of species i in Water (mole/m3)
VPi = Pure Component Vapor Pressure (atm)
To utilize any other set of units, it becomes necessary to develop the appropriate conversion factors and to build them into the above equation. For example, it is often convenient to utilize Vw in (cm3 /mole) and Si in wt% (gm/100 gms solution).
The conversions are as follows: Vw (m3/mole) = 10-6 * Vw (cm3/mole) Si (mole/m3) = (104
* RHOw / MWi) * Si (gm/100 gm H2O)
Note that for sparingly soluble substances in water, 100 gms solution is, in the limit, the same as 100 gms of H2O. Thus, the following formula: i i p w i p K = 55.51*V * S V KPi = 0.5551 * Vw * Si * RHOw / (VPi * MWi) should be used in conjunction with the following set of units:
Entity Units KPi atm Vw cm3/gmole Si gm/100 gm H2O RHOw gm H2O/cm3 VPi atm MWi gm/gmole
A further simplification can be made by observing that Vw * RHOw in these units is exactly 18.015 and thus the equation can be written: Thus, based upon this relationship, the ProChem K(T) can be developed. If we now recognize that the VLE K is simply related to the difference in the free energy between the vapor and the aqueous molecular species, we can use available thermodynamics for the vapor phase to back out the free energy for the aqueous molecule. Once we have the free energy of the aqueous molecule as a function of temperature, we can do a linear regression for the Helgeson omega, c1 and c2 coefficients. The reference state free energy for the aqueous molecule can be obtained directly from the knowledge of the VLE K at the reference state and the free energy of the vapor at this temperature. The HREF for the aqueous molecule can be obtained from the Van Hoff equation which relates the difference the reference state enthalpy for the vapor and aqueous molecule to the first derivative of the VLE K(T) with respect to temperature. Finally, the SREF for the aqueous molecule can be obtained from the third law of thermodynamics since both vapor and aqueous molecule free energy and enthalpy are known at that point. Derivation of Equation (1) The starting point in derivation of equation (1) is based upon the definition of equilibrium which is that the chemical potential, or Gibbs Free Energy of each species in the vapor phase and aqueous ( ) i i p i p i K = 10* S V * MW phase is equal. Specifically:
Gi(VAP) = Gi(AQ)
In each case, the Gibbs Free Energy can be expressed by the sum of a standard state contribution and an excess contribution: where: Gi = Gibbs Free Energy Gi' = Standard State Gibbs Free Energy R = Gas Constant T = Absolute Temperature fi = Fugacity (activity) Coefficient VAP = Vapor Phase AQ = Aqueous Phase pi = Partial Pressure mi = Molality in the Aqueous Phase Rearranging terms we get:
(3)
We now make two assumptions upon which the rest of the derivation is based: (1) - The organic i is sparingly soluble in water. i (vap) i(vap) ′ ′ i i (aq) i(aq) G +RTln( F * p )=G +RTln( i f * m ) i p i(aq) i (vap) i(aq) i i(vap) i K = ( G -G RT = f * m ( f * p ) exp ⎛ ′
⎟ ′
(2) - Water is sparingly soluble in the organic liquid.
Ref: Marshall Rafal Subject: Development of ProChem K-Values For Sparingly Soluble Organics OLI Internal Report 90/1 Date: 11/1/90 (Revision #1 - 1/22/92)