Difference between revisions of "Calcium Carbonate Solubility"
(Created page with "The solubility of calcite (CaCO<sub>3</sub>, Calcite) seems to show an inverse solubility with respect to temperature. This seems contradictory to what is normally observed i...") |
(No difference)
|
Revision as of 07:08, 7 January 2016
The solubility of calcite (CaCO3, Calcite) seems to show an inverse solubility with respect to temperature. This seems contradictory to what is normally observed in the field.
The confusion occurs because of the assumed presence of carbon dioxide (CO2). Normally atmospheric air contains approximately 300 ppm CO2. However, when initially using the OLI software such as the OLI Studio program, the user often forgets to add the CO2 to the calculation.
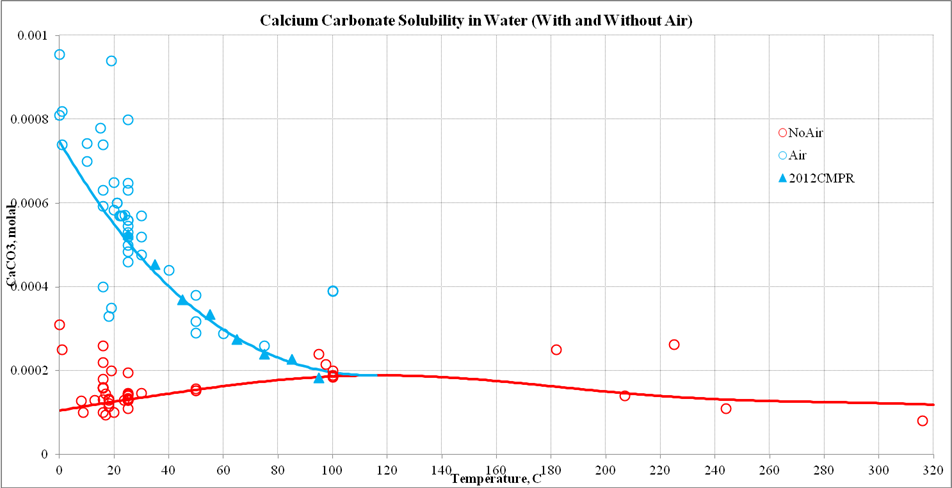
Here is a plot of CaCO3 solubility versus temperature with and without CO2:
In the case of no air, there is a slight increase in the solubility of CaCO3 with respect to temperature although there is significant scatter to the data. This is what is reported.
With atmospheric CO2 present, the decrease in solubility is quite pronounced. A recent paper in 2012 also shows this trend. I think in some earlier experiments, the amount of atmospheric CO2 was not recorded which gave the decrease in solubility which we all expected.
References: