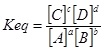Difference between revisions of "ESP Examples: Using Constrained Reaction Kinetics"
(Created page with "Overview The explanation of how to use reaction kinetics in ESP has been described in the OLI Engine Manual and in OLI Tips 51 . Frequently a user wants to describe...") |
|||
| Line 6: | Line 6: | ||
To briefly explain the procedure we first need to look at a generic equilibrium reaction: | To briefly explain the procedure we first need to look at a generic equilibrium reaction: | ||
| + | |||
| + | [[File:Editing ESP Image 1.jpg]] | ||
| + | |||
| + | The standard equilibrium constant expression is<sup>3</sup> : | ||
| + | |||
| + | [[File:Editing ESP Image 2.jpg]] | ||
| + | |||
| + | The forward rate [[File:Editing ESP Image 3.jpg]] is: | ||
| + | |||
| + | [[File:Editing ESP Image 4.jpg]] | ||
| + | |||
| + | And the reverse rate [[File:Editing ESP Image 5.jpg]] is: | ||
| + | |||
| + | [[File:Editing ESP Image 6.jpg]] | ||
| + | |||
| + | <sup>1</sup>This manual is for version 6.7 of OLI/Engine. | ||
| + | |||
| + | <sup>2</sup>You can click these links for the reference material. | ||
| + | |||
| + | <sup>3</sup>We are ignoring activity coefficients to simplify the example. | ||
Revision as of 09:39, 14 July 2016
Overview
The explanation of how to use reaction kinetics in ESP has been described in the OLI Engine Manual and in OLI Tips 51 .
Frequently a user wants to describe a chemical reaction in terms of reaction kinetics rather than equilibrium. The user may also want to constrain the reaction kinetics such that the forward and reverse rates of reaction do not exceed the limits placed on it by chemical equilibrium.
To briefly explain the procedure we first need to look at a generic equilibrium reaction:
The standard equilibrium constant expression is3 :
1This manual is for version 6.7 of OLI/Engine.
2You can click these links for the reference material.
3We are ignoring activity coefficients to simplify the example.