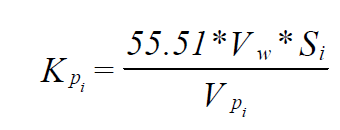Difference between revisions of "Sparingly Soluble Organics"
| Line 11: | Line 11: | ||
V<sub>w<sub> = Molar Volume of Water (m<sup>3<sub>/mole) | V<sub>w<sub> = Molar Volume of Water (m<sup>3<sub>/mole) | ||
| − | S<sub>i<sub> = | + | S<sub>i<sub> = Solubility of species i in Water (mole/m<sup>3<sup>) |
| + | |||
| + | VP<sub>i<sub> = Pure Component Vapor Pressure (atm) | ||
| + | |||
| + | To utilize any other set of units, it becomes necessary to develop the appropriate conversion factors and to build them into the above equation. For example, it is often convenient to utilize V<sub>w<sub> in cm<sup>3<sup>/mole and S<sub>i<sub> in wt% (gm/100 gms solution). | ||
Revision as of 11:39, 24 March 2016
The starting point for developing of K-Values for sparingly soluble organics is the following relationship developed in the Transactions of the 11th User Conference for OLI Systems Inc.
Where:
Kpi = ProChem K-Value for species i (1/atm)
Vw = Molar Volume of Water (m3/mole)
Si = Solubility of species i in Water (mole/m3)
VPi = Pure Component Vapor Pressure (atm)
To utilize any other set of units, it becomes necessary to develop the appropriate conversion factors and to build them into the above equation. For example, it is often convenient to utilize Vw in cm3/mole and Si in wt% (gm/100 gms solution).